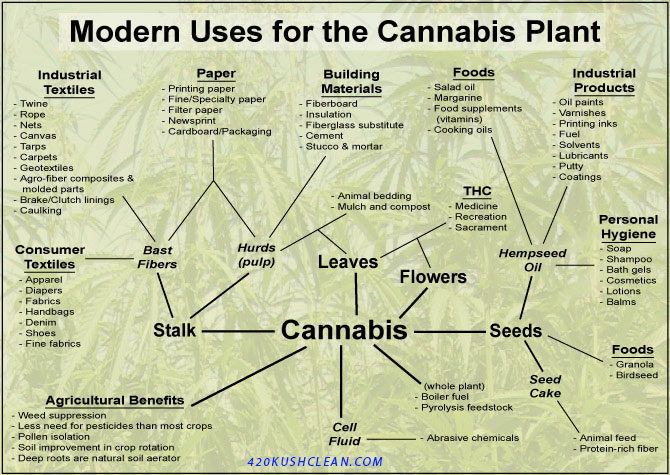
UVB and THC Production
Pate, D.W., 1994. Chemical ecology of Cannabis. Journal of the International Hemp Association 2: 29, 32-37.
The production of cannabinoids and their associated terpenes in Cannabis is subject to environmental influences as well as hereditary determinants. Their biosynthesis occurs in specialized glands populating the surface of all aerial structures of the plant. These compounds apparently serve as defensive agents in a variety of antidessication, antimicrobial, antifeedant and UV-B pigmentation roles. In addition, the more intense ambient UV-B of the tropics, in combination with the UV-B lability of cannabidiol, may have influenced the evolution of an alternative biogenetic route from cannabigerol to tetrahydrocannabinol in some varieties.
Ultraviolet radiation
Another stress to which plants are subject results from their daily exposure to sunlight. While necessary to sustain photosynthesis, natural light contains biologically destructive ultraviolet radiation. This selective pressure has apparently affected the evolution of certain defenses, among them, a chemical screening functionally analogous to the pigmentation of human skin. A preliminary investigation (Pate 1983) indicated that, in areas of high ultraviolet radiation exposure, the UV-B (280-315 nm) absorption properties of THC may have conferred an evolutionary advantage to Cannabis capable of greater production of this compound from biogenetic precursor CBD. The extent to which this production is also influenced by environmental UV-B induced stress has been experimentally determined by Lydon et al. (1987). Their experiments demonstrate that under conditions of high UV-B exposure, drug-type Cannabis produces significantly greater quantities of THC. They have also demonstrated the chemical lability of CBD upon exposure to UV-B (Lydon and Teramura 1987), in contrast to the stability of THC and CBC. However, studies by Brenneisen (1984) have shown only a minor difference in UV-B absorption between THC and CBD, and the absorptive properties of CBC proved considerably greater than either. Perhaps the relationship between the cannabinoids and UV-B is not so direct as first supposed. Two other explanations must now be considered. Even if CBD absorbs on par with THC, in areas of high ambient UV-B, the former compound may be more rapidly degraded. This could lower the availability of CBD present or render it the less energetically efficient compound to produce by the plant. Alternatively, the greater UV-B absorbency of CBC compared to THC and the relative stability of CBC compared to CBD might nominate this compound as the protective screening substance. The presence of large amounts of THC would then have to be explained as merely an accumulated storage compound at the end of the enzyme-mediated cannabinoid pathway. However, further work is required to resolve the fact that Lydon’s (1985) experiments did not show a commensurate increase in CBC production with increased UV-B exposure.
This CBC pigmentation hypothesis would imply the development of an alternative to the accepted biochemical pathway from CBG to THC via CBD. Until 1973 (Turner and Hadley 1973), separation of CBD and CBC by gas chromatography was difficult to accomplish, so that many peaks identified as CBD in the preceding literature may in fact have been CBC. Indeed, it has been noted (De Faubert Maunder 1970) and corroborated by GC/MS (Turner and Hadley 1973) that some tropical drug strains of Cannabis do not contain any CBD at all, yet have an abundance of THC. This phenomenon has not been observed for northern temperate varieties of Cannabis. Absence of CBD has led some authors (De Faubert Maunder 1970, Turner and Hadley 1973) to speculate that another biogenetic route to THC is involved. Facts scattered through the literature do indeed indicate a possible alternative. Holley et al. (1975) have shown that Mississippi-grown plants contain a considerable content of CBC, often in excess of the CBD present. In some examples, either CBD or CBC was absent, but in no case were plants devoid of both. Their analysis of material grown in Mexico and Costa Rica served to accentuate this trend. Only one example actually grown in their respective countries revealed the presence of any CBD, although appreciable quantities of CBC were found. The reverse seemed true as well. Seed from Mexican material devoid of CBD was planted in Mississippi and produced plants containing CBD.
Could CBC be involved in an alternate biogenetic route to THC? Yagen and Mechoulam (1969) have synthesized THC (albeit in low yield) directly from CBC. The method used was similar to the acid catalyzed cyclization of CBD to THC (Gaoni and Mechoulam 1966). Reaction by-products included cannabicyclol, delta-8-THC and delta-4,8-iso-THC, all products which have been found in analyses of Cannabis (e.g., Novotny et al. 1976). Finally, radioisotope tracer studies (Shoyama et al. 1975) have uncovered the intriguing fact that radiolabeled CBG fed to a very low THC-producing strain of Cannabis is found as CBD, but when fed to high THC-producing plants, appeared only as CBC and THC. Labeled CBD fed to a Mexican example of these latter plants likewise appeared as THC. Unfortunately, radiolabeled CBC was not fed to their plants, apparently in the belief that CBC branched off the biogenetic pathway at CBD and dead ended. Their research indicated that incorporation of labeled CBG into CBD or CBC was age dependent. Vogelman et al. (1988) likewise report that the developmental stage of seedlings, as well as their exposure to light, affects the occurrence of CBG, CBC or THC in Mexican Cannabis. No CBD was reported.
UVB LIGHT
Ultra-violet B light is a spectrum of light that is invisible to us but is visible to insects and some other organisms. In humans it causes suntan and sunburn and is implicated in the formation of eye cataracts. It is the light emitted by tanning bulbs.
UVB light also affects marijuana potency. The potency of high quality marijuana increases in direct ratio to the amount of UVB light it receives. This is very significant. In California, where the medical dispensaries operate in an unrestricted market; many dispensaries reject fall harvested outdoor material as inferior. They have found it lacks the potency of indoor crops and is a harsh smoke. However, when they were presented with marijuana grown outdoors but forced to ripen August 10, they accepted it as if it were indoor because of its high potency and lack of harshness. I think the harshness results from cool nights.
Indoors, under fluorescent and HPS lamps, gardens receive little UV-B light. Metal halides emit a bit more. However, there are ways of supplying your garden with UV-B light. Tanning lamps work, that is, lamps that tan people, because of the UV-B light they emit. Using tanning lamps will increase the THC content of the crop. Reptiles and lizards require the spectrum to stay healthy. So the spectrum usually comprises about 10 percent of their output. If you want to try tanning lamps they are available on the Internet. Use between 5-10 percent of your total wattage to these lamps. For a 1000-watt garden use 100 watts of special lighting. The Solis-Tek 10K metal halide bulb emits a tremendous amount of UVB and measured with a specific UVB meter, the SolarMeter 6.2,
Adding UV-B light to your garden will enhance your marijuana naturally, without “special formulas” and chemicals.
Conclusions
Although the chemistry of Cannabis has come under extensive investigation, more work is needed to probe the relationship of its resin to biotic and abiotic factors in the environment. Glandular trichomes are production sites for the bulk of secondary compounds present. It is probable that the cannabinoids and associated terpenes serve as defensive agents in a variety of antidessication, antimicrobial, antifeedant and UV-B pigmentation roles. UV-B selection pressures seem responsible for the distribution of THC-rich Cannabis varieties in areas of high ambient radiation, and may have influenced the evolution of an alternate biogenetic pathway from CBG to THC in some of these strains. Though environmental stresses appear to be a direct stimulus for enhanced chemical production by individual plants, it must be cautioned that such stresses may also skew data by hastening development of the highly glandular flowering structures. Future studies will require careful and representative sampling to assure meaningful results.
References
- Abel E., 1980. Marihuana: The first 12,000 years. Plenum Press, New York.
- Adams R. and C.K. Caine, W.D. McPhee and T.N. Wearn, 1941. Structure of cannabidiol. XII. Isomerization to tetrahydro-cannabinols. Journal of the American Chemical Society 63: 2209-2213.
- Adams Jr., T.C. and L.A. Jones, 1973. Long chain hydrocarbons of Cannabis and its smoke. Agr. Food Chemistry 21: 1129-1131.
- Bazzaz F.A., D. Dusek, D.S. Seigler and A.W. Haney, 1975. Photosynthesis and cannabinoid content of temperate and tropical populations of Cannabis sativa. Biochemical Systematics and Ecology 3: 15-18.
- Binder M., 1976. Microbial transformation of (-)-delta-3,4-trans-tetrahydrocannabinol by Cunninghamella blakesleena Lender. Helvetica Chimica Acta 63: 1674-1684.
- Binder M. and A. Popp, 1980. Microbial transformation on cannabinoids. Part 3: major metabolites of (3R, 4R)-delta-1-tetrahydrocannabinol. Helvetica Chimica Acta 2515-2518.
- Boucher F., L. Cosson, J. Unger and M.R. Paris, 1974. Le Cannabis sativa L.; races chemiques ou varietes. Pl. Med. Phytotherap. 8: 20-31.
- Bouquet J., 1950. Cannabis. UN Bulletin on Narcotics 2: 14-30.
- Braut-Boucher F., 1980. Effet des conditions ecophysiologiques sur la croissance, le developpement et le contenu en cannabinoides de clones correspondant aux deux types chimiques du Cannabis sativa L. originaire d’Afrique du Sud. Physiol. Veg. 18: 207-221.
- Brenneisen R., 1984. Psychotrope Drogen II. Bestimmung der Cannabinoid in Cannabis sativa L. und in Cannabisprodukten mittels Hochdruckflussigkeitschromatographie (HPLC). Pharm. Acta Helv. 59: 247-259.
- Briosi G. and F. Tognini, 1894. Anatomia della canapa. Parte prima: Organi sessuali. Atti. Ist. Bot. Pavia Ser. II. 3: 91-209.
- Charles,V. and A. Jenkins, 1914. A fungous disease of hemp. J. Agric. Res. 3: 81-85.
- Coffman C.B. and W.A. Gentner, 1975. Cannabinoid profile and elemental uptake of Cannabis sativa L. as influenced by soil characteristics. Agronomy Journal 67: 491-497.
- Dayanandan P. and P.B. Kaufman, 1976. Trichomes of Cannabis sativa L. (Cannabaceae). American Journal of Botany 63: 578-591
- De Faubert Maunder M.J., 1970. A comparative evaluation of the tetrahydrocannabinol content of Cannabis plants. J. Ass. Pub. Anal. 8: 42-47.
- De Faubert Maunder M.J., 1976. The forensic significance of the age and origin of Cannabis. Med. Sci. Law 16: 78-89.
- De Meijer E.P.M., H.J. Van der Kamp and F.A. Van Eeuwijk. Characterization of Cannabis accessions with regard to cannabinoid content in relation to other plant characters. Euphytica 62: 187-200.
- De Pasquale A., G. Tumino and R.C. De Pasquale, 1974. Micromorphology of the epidermic surfaces of female plants of Cannabis sativa L. UN Bulletin on Narcotics 26: 27-40
- De Zeeuw R.A., Th. M. Malingre and F.W.H.M. Merkus, 1972a. Tetrahydrocannabinolic acid, an important component in the evaluation of Cannabis products. J. Pharm. Pharmacology 24: 1-6.
- De Zeeuw R.A., T.B. Vree, D.D. Breimer and C.A.M. Van Ginnekin, 1973a. Cannabivarichromene, a new cannabinoid with a propyl side chain in Cannabis. Experientia 29: 260-261.
- De Zeeuw R.A., J. Wijsbek, D.D. Breimer, T.B. Vree, C.A. Van Ginneken and J.M. van Rossum, 1972b. Cannabinoids with a propyl side chain in Cannabis. Occurence and chromatographic behavior.Science 175: 778-779.
- De Zeeuw R.A., J. Wijsbek and Th.M. Malingre, 1973b. Interference of alkanes in the gas chromatographic analysis of Cannabis products. J. Pharm. Pharmacology 25: 21-26.
- Eisner T., 1970. Chemical defense against predation in arthropods. Page 127 in E. Sondheimer and J.B. Simone, eds., Chemical ecology, Academic Press, New York.
- ElSohly M.A. and C.E. Turner, 1976. A review of nitrogen containing compounds from Cannabis sativa L. Pharmaceutisch Weekblad III: 1069-1075.
- ElSohly H., C.E. Turner, A.M. Clark and M.A. ElSohly, 1982. Synthesis and antimicrobial properties of certain cannabichrome and cannabigerol related compounds. Journal of the Pharmaceutical Sciences71: 1319-1323.
- Emboden W.A., 1972. Ritual use of Cannabis sativa L.: a historical-ethnographic survey. Page 224 in P. Furst, ed., Flesh of the gods, Praeger Press, New York.
- Fairbairn J.W., 1972. The trichomes and glands of Cannabis sativa L. UN Bulletin on Narcotics 24: 29-33.
- Farkas J., and E. Andrassy, 1976. The sporostatic effect of cannabidiolic acid. Acta Alimentaria 5: 57-67.
- Ferenczy L., 1956. Antibacterial substances in seeds of Cannabis. Nature 178: 639.
- Ferenczy L., L. Grazca and I. Jakobey, 1958. An antibacterial preparation from hemp (Cannabis sativa L.). Naturwissenschaften 45: 188.
- Fetterman P.S., N.J. Doorenbos, E.S. Keith and M.W. Quimby, 1971a. A simple gas liquid chromatography procedure for determination of cannabinoidic acids in Cannabis sativa L. Experientia 27: 988-90.
- Fetterman P.S., E.S. Keith, C.W. Waller, O. Guerrero, N.J. Doorenbos and M.W. Quimby, 1971b. Mississippi-grown Cannabis sativa L.: Preliminary observation on chemical definition of phenotype and variations in tetrahydrocannabinol content versus age, sex, and plant part. Journal of the Pharmaceutical Sciences 60: 1246-1249.
- Fetterman P.S. and C.E. Turner, 1972. Constituents of Cannabis sativa L. I. Propyl homologs of cannabinoids from an Indian variant. Journal of the Pharmaceutical Sciences 61: 1476-1477.
- Gal I.E. and O. Vajda, 1970. Influence of cannabidiolic acid on microorganisms. Elelmez. Ipar. 23: 336-339.
- Gaoni Y. and R. Mechoulam, 1966. Hashish, VII. The isomerization of cannabidiol to tetrahydrocannabinols. Tetrahedron 22: 1481-1488.
- Gaoni Y. and R. Mechoulam, 1971. The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. Journal of the American Chemical Society 93: 217-224.
- Garrett E.R. and C.A. Hunt, 1974. Physico-chemical properties, solubility and protein binding of delta-9-tetrahydrocannabinol. Journal of the Pharmaceutical Sciences 63: 1056-1064.
- Gellert M., I. Novak, M. Szell and K. Szendrei, 1974. Glycosidic components of Cannabis sativa L. I. Flavonoids. UN Document ST/SOA/SER.S/50 Sept. 20.
- Gill E.W., 1971. Propyl homologue of tetrahydrohcannabinol: Its isolation from Cannabis, properties and synthesis. Journal of the Chemical Society p. 579-82.
- Gill E.W., W.D.M. Paton and R.G. Pertwee, 1970. Preliminary experiments on the chemistry and pharmacology of Cannabis. Nature 228: 134-136.
- Grlic L. and A. Andrec, 1961. The content of acid fraction in Cannabis resin of various age and provenance. Experientia 17: 325-326.
- Hakim H.A., Y.A. El Kheir and M.I. Mohamed, 1986. Effect of climate on the content of a CBD-rich variant of Cannabis. Fitoterapia 57: 239-241.
- Hammond C.T. and P.G. Mahlberg, 1973. Morphology of glandular hairs of Cannabis sativa from scanning electron microscopy. American Journal of Botany 60: 524-528.
- Hammond C.T. and P.G. Mahlberg, 1978. Ultrastructural development of capitate glandular hairs of Cannabis sativa L. (Cannabaceae). American Journal of Botany 65: 140-151.
- Hammond C.T. and P.G. Mahlberg, 1994. Phloroglucinol glucoside as a natural constituent of Cannabis sativa. Phytochemistry 37: 755-756.
- Haney A. and F.A. Bazzaz, 1970. Discussion in The botany and chemistry of Cannabis. Joyce, C.R.B. and S.H. Curry, eds. Churchill, London.
- Haney A. and B.B. Kutscheid, 1973. Quantitative variation in chemical constituents of marihuana from stands of naturalized Cannabis sativa L. in east central Illinois. Economic Botany 27: 193-203.
- Haney A. and B.B. Kutscheid, 1975. An ecological study of naturalized hemp (Cannabis sativa L.) in east-central Illinois. American Midland Naturalist 93: 1-24.
- Hanus I., 1975a. The present state of knowledge in the chemistry of substances of Cannabis sativa L. III. Terpenoid substances. Acta Universitatis Palackianae Olomucensis Facultatis Medicae 73: 233-239.
- Hanus I., 1975b. The present state of knowledge in the chemistry of substances of Cannabis sativa L. IV. Nitrogen containing compounds. Acta Universitatis Palackianae Olomucensis Facultatis Medicae73: 241-244.
- Hanus I., 1976a. The present state of knowledge in the chemistry of substances of Cannabis sativa L. V. Addendum to part I-IV. Acta Universitatis Palackianae Olomucensis Facultatis Medicae 76: 153-166.
- Hanus I., 1976b. The present state of knowledge in the chemistry of substances of Cannabis sativa L. VI. The other contained substances. Acta Universitatis Palackianae Olomucensis Facultatis Medicae76: 167-173.
- Harvey O.J., 1976. Characterization of the butyl homologs of delta-1-tetrahydrocannabinol and cannabidiol in samples of Cannabis by combined gas chromatography and mass spectrometry. J. Pharm. Pharmacol. 28: 280-285.
- Hendricks H., T.M. Malingre, S. Batterman and R. Bos, 1975. Mono- and sesquiterpene hydrocarbons of the essential oil of Cannabis sativa. Phytochemistry 14: 814-15.
- Holley J. H., K.W. Hadley and C.E. Turner, 1975. Constituents of Cannabis sativa L. XI. Cannabidiol and cannabichromene in samples of known geographical origin. Journal of the Pharmaceutical Sciences 64: 892-895.
- Honma S., H. Kaneshima, M. Mori,and T. Kitsutaka, 1971a. Cannabis grown in Hokkaido. 2. Contents of cannabinol, tetrahydrocannabinol and cannabidiol in wild Cannabis. Hokkaidoritsu Eisei Kenkyushoho 21: 180-185.
- Honma S., H. Kaneshima, M. Mori and T. Kitsutaka, 1971b. Cannabis grown in Hokkaido. 3. Variation in the amount of narcotic components of Cannabis and its growth. Hokkaidoritsu Eisei Kenkyushoho21: 186-190.
- Hood L.V.S., M.E. Dames and G.T. Barry, 1973. Headspace volatiles of marijuana. Nature 242: 402-403.
- Isbell H., 1973. Research on Cannabis (marijuana). UN Bulletin on Narcotics 25: 37-48.
- Kabelik J., Z. Krejci and F. Santavy, 1960. Cannabis as a medicament. UN Bulletin on Narcotics 12: 5-23.
- Kaneshima H., M. Mori and N. Mizuno, 1973. Studies on Cannabis in Hokkaido (Part 6). The dependence of Cannabis plants on iron nutrition. Hokkaidoritsu Eisei Kenkyusho 23: 3-5.
- Khare B.P., S.B. Gupta and S. Chandra, 1974. Biological efficacy of some plant materials against Sitophilus oryzae Linneaeous. Indian Journal of Agricultural Research 8: 243-248.
- Kimura M. and K. Okamoto, 1970. Distribution of tetrahydrocannabinolic acid in fresh wild Cannabis. Experientia 26: 819-20.
- Krejci Z., 1970. Changes with maturation in amounts of biologically interesting substances of Cannabis. Page 49 in The botany and chemistry of Cannabis. Joyce, C.R.B. and S.H. Curry, eds. Churchill, London.
- Lanyon V.S., J.C. Turner and P.G. Mahlberg, 1981. Quantitative analysis of cannabinoids in the secretory product from captitate-stalked glands of Cannabis sativa L. (Cannabaceae). Botanical Gazette142: 316-319.
- Latta R.P. and B.J. Eaton, 1975. Seasonal fluctuations in cannabinoid content of Kansas marijuana. Economic Botany 29: 153-163.
- Ledbetter M.C. and A.D. Krikorian, 1975. Trichomes of Cannabis sativa as viewed with scanning electron microscope. Phytomorphology 25: 166-176.
- Lentz P.L., C.E. Turner, L.W. Robertson and W.A. Gentner, 1974. First North American record for Cercospora cannabina, with notes on the identification of C. cannabina and C. cannabis. Plant Disease Reporter 58: 165-168.
- Levin D.A., 1973. The role of trichomes in plant defense. Quarterly Review of Biology 48: 3-16.
- Lydon J., 1985. The effects of Ultraviolet-B radiation on the growth, physiology and cannabinoid production of Cannabis sativa L. Ph.D. Dissertation, University of Maryland.
- Lydon J. and A.H. Teramura, 1987. Photochemical decomposition of cannabidiol in its resin base. Phytochemistry 26: 1216-1217.
- Lydon J., A.H. Teramura and C.B. Coffman, 1987. UV-B radiation effects on photosynthesis, growth and cannabinoid production of two Cannabis sativa chemotypes. Photochemistry and Photobiology 46: 201-206.
- Mahlberg P.G. and E.-S. Kim, 1992. Secretory vesicle formation in glandular trichomes of Cannabis sativa (Cannabaceae). American Journal of Botany 79: 166-173.
- Malingre T.N., H. Hendricks, S. Batterman, R. Bos and J. Visser, 1975. The essential oil of Cannabis sativa.. Planta Medica 28: 56-61.
- Marshman J., C.D. Yawney and R.E. Popham, 1976. A note on the cannabinoid content of Jamaican ganja. UN Bulletin on Narcotics 28: 63-68.
- Martin L., D.M. Smith and C.G. Farmilo, 1961. Essential oil from fresh Cannabis sativa and its use in identification. Nature 191: 774-776.
- Masoud A.N. and N.J. Doorenbos, 1973. Mississippi grown Cannabis sativa L. III. Cannabinoid and cannabinoic acid content. Journal of the Pharmaceutical Sciences 62: 313-315.
- McCain A.H. and C. Noviello, 1985. Biological control of Cannabis sativa. Pages 635-642 in Proceedings of the sixth international symposium on biological control of weeds. Delfosse, E.S., ed. Agricultural Canada, Ottawa, Canada.
- McPartland J.M., 1984. Pathogenicity of Phomopsis ganjae on Cannabis sativa and the fungistatic effect of cannabinoids produced by the host. Mycopathologia 87: 149-154.
- Mechoulam R., 1970. Marijuana chemistry. Science 168: 1159-1166.
- Mechoulam R. and Y. Gaoni, 1965. Hashish IV. The isolation of cannabinolic, cannabidiolic and cannabigerolic acids. Tetrahedron 21: 1223-1229.
- Merkus F.W.H.M., 1971. Two new constituents of hashish. Nature 232: 579-580.
- Mikuriya T.H., 1969. Marijuana in medicine: past, present and future. California Medicine 110: 34-40.
- Mobarak Z., D. Bieniek and F. Korte, 1974a. Studies on non-cannabinoids of hashish. Isolation and identification of some hydrocarbons. Chemosphere 3: 5-8.
- Mobarak Z., D. Bieniek and F. Korte, 1974b. Studies on non-cannabinoids of hashish II. An approach to correlate the geographical origin of Cannabis with hydrocarbon content by chromatographic analysis.Chemosphere 3: 265-70.
- Muller W.H. and R. Hauge, 1967. Volatile growth inhibitors produced by Salvia leucophylla: effect on seedling anatomy. Bulletin of the Torrey Botanical Club 94: 182-190.
- Muller C.H., W.H. Muller and B.L. Haines 1964. Volatile growth inhibitors produced by aromatic shrubs. Science 143: 471-73.
- Murari G., S. Lombardi, A.M. Puccini and R. De Sanctis, 1983. Influence of environmental conditions on tetrahydrocannabinol (delta-9-THC) in different cultivars of Cannabis sativa L. Fitoterapia 54: 195-201.
- Novotny M., M.L. Lee, C.-E. Low and A. Raymond, 1976. Analysis of marijuana samples from different origins by high-resolution gas-liquid chromatography for forensic application. Analytical Chemistry 48: 24-29.
- Ohlsson A., C.I. Abou-Chaar, S. Agurell, I.M. Nilsson, K. Olofsson and F. Sandberg, 1971. Cannabinoid constituents of male and female Cannabis sativa. UN Bulletin on Narcotics 23: 29-32.
- Ono M., M. Shimamine and K. Takahashi, 1972. Studies on Cannabis. III. Distribution of tetrahydrocannabinol in the Cannabis plant. Eisei Shikenjo Hokoku 90: 1-4.
- Paris M., F. Boucher and L. Cosson, 1975a. The constituents of Cannabis sativa pollen. Economic Botany 29: 245-253.
- Paris R.R., E. Henri and M. Paris, 1975b. O c-flavonoidima Cannabis sativa L. Arh. Farmaciju 25: 319-28.
- Paris R.R. and M.R. Paris, 1973. Sur les flavonoides du chanvre (Cannabis sativa L.). Comptes Rendus Acad. Sci. Ser. D. 277: 2369-71.
- Pate D.W., 1983. Possible role of ultraviolet radiation in evolution of Cannabis chemotypes. Economic Botany 37: 396-405.
- Radosevic A., M. Kupinic and Lj. Grlic, 1962. Antibiotic activity of various types of Cannabis resin. Nature 195: 1007-1009.
- Robertson L.W., M.A. Lyle and S. Billets, 1975. Biotranformation of cannabinoids by Syncephalastrum racemosum.. Biomedical Mass Spectroscopy 2: 266-271.
- Rothschild M., M.G. Rowen and J.W. Fairbairn, 1977. Storage of cannabinoids by Arctia caja and Zonocerus elegans fed on chemically distinct strains of `Cannabis sativa. Nature 266: 650-651.
- Rothschild M. and J.W. Fairbairn, 1980. Ovipositing butterfly (Pieris brassicae L.) distinguishes between aqueous extracts of two strains of Cannabis sativa L. and THC and CBD. Nature 286: 56-59.
- Sharma G.K., 1975. Altitudinal variation in leaf epidermal patterns of Cannabis sativa. Bulletin of the Torrey Botanical Club 102: 199-200.
- Shoyama Y., H. Hirano and I. Nishioka, 1984. Biosynthesis of Propyl Cannabinoid Acid and Its Biosynthetic Relationship with Pentyl and Methyl Cannabinoid Acids. Phytochemistry 29: 1909-1912.
- Shoyama Y., M. Yagi, I. Nishioka, and T. Yamauchi, 1975. Biosynthesis of cannabinoid acids. Phytochemistry 14: 2189-2192.
- Shukla D.D. and V.N. Pathak, 1967. A new species of Ascochyta on Cannabis sativa L. Sydowia Annals of Mycology 21: 277-278.
- Small E. and H.D. Beckstead, 1973. Common cannabinoid phenotypes in 350 stocks of Cannabis. Lloydia 36: 144-165.
- Smith G.E. and A. Haney, 1973. Grapholitha tristrigana (Clemens) (Lepidoptera: Tortricidae) on naturalized hemp (Cannabis sativa L.) in east-central Illinois. Transactions of the Illinois State Academy of Sciences 66: 38-41.
- Srivastava S.L. and S.C. Naithani, 1979. Cannabis sativa Linn., a new host for Phoma sp. Current Science of India 48: 1040-1005.
- Stannard L.J., J.R. Dewitt and T.C. Vance, 1970. The marijuana thrips, Oxythrips cannabensis, a new record for Illinois and North America. Transactions of the Illinois Academy of Sciences 63: 152-156.
- Steinberg S., J. Offermeier, B.I. Field and F.W. Jansen Van Ryssen, 1975. Investigation of the influence of soil types, environmental conditions, age and morphological plant parts on the chemical composition ofCannabis sativa (Dagga) plants. South African Medical Journal 45: 279.
- Turner C.E., M.A. ElSohly and E.G. Boeren, 1980. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. Journal of Natural Products 43: 169-234.
- Turner C.E. and K. Hadley, 1973. Constituents of Cannabis sativa L. II. Absence of cannabidiol in an African variant. Journal of the Pharmaceutical Sciences 62: 251-255.
- Turner C.E., K. Hadley and P.S. Fetterman, 1973a. Constituents of Cannabis sativa L. VI: Propyl homologs in samples of known geographic origin. Journal of the Pharmaceutical Sciences 62: 1739-1741.
- Turner C.E., K.W. Hadley, P.S. Fetterman, N.J. Doorenbos , M.W. Quimby and C. Waller, 1973b. Constituents of Cannabis sativa L. IV: Stability of cannabinoids in stored plant material. Journal of the Pharmaceutical Sciences 62: 1601-1605.
- Turner J.C., J.K. Hemphill and P.G. Mahlberg, 1978. Cannabinoid composition and gland distribution in clones of Cannabis sativa L. (Cannabaceae). UN Bulletin on Narcotics 30: 55-65.
- Turner J.C. and Mahlberg, P.G., 1988. In vivo incorporation of labeled precursors into cannabinoids in seedlings of Cannabis sativa L. (Cannabaceae). Pages 263-270 in Chesher, G., P. Consroe and R. Musty, eds. Marihuana. Australian Government Publications, Canberra.
- Van Klingeren B. and M. Ten Ham, 1976. Antibacterial activity of delta-9-tetrahydrocannabinol and cannabidiol. Antonie van Leeuwenhoek Journal of Microbiology and Serology 42: 9-12.
- Vogelmann A.F., J.C. Turner and P.G. Mahlberg, 1988. Cannabinoid composition in seedlings compared to adult plants of Cannabis sativa. Journal of Natural Products 51: 1075-1079.
- Vree T.B., D.D. Breimer, C.A.M. Van Gienneken and J.M. Rossum, 1971. Identification of methyl and homologs of CBD, THC and CBN in hashish by a new method of combined gas chromatography-mass spectrometry. Acta Pharm. Sue. 8: 683-84.
- Vree T.B., D.D. Breimer, C.A.M. Van Gienneken and J.M. Rossum, 1972a. Identification of cannabicyclol with a pentyl or propyl side-chain by means of combined gas chromatography-mass spectrometry.Journal of Chromatography 74: 124-127.
- Vree T.B., D.D. Breimer, C.A.M. Van Gienneken and J.M. Rossum, 1972b. Identification in hashish of tetrahydrocannabinol, cannabidiol and cannabinol analogs with methyl side-chain. J. Pharm. Pharmacol.24: 7-12.
- Yagen B. and R. Mechoulam, 1969. Stereospecific cyclizations and isomerizations of cannabichromene and related cannabinoids. Tetrahedron Letters 60: 5356-5363.
 The federal government allowed Colorado’s and Washington’s historic marijuana laws to take effect last year. President Barack Obama signed the 2014 farm bill, which legalized industrial hemp production for research purposes in the states that permit it, and the first hemp crops in U.S. soil in decades are already growing. And in May, the U.S. House passed measures attempting to limit Drug Enforcement Administration crackdowns on medical marijuana shops when they’re legal in a state.
The federal government allowed Colorado’s and Washington’s historic marijuana laws to take effect last year. President Barack Obama signed the 2014 farm bill, which legalized industrial hemp production for research purposes in the states that permit it, and the first hemp crops in U.S. soil in decades are already growing. And in May, the U.S. House passed measures attempting to limit Drug Enforcement Administration crackdowns on medical marijuana shops when they’re legal in a state.